when an atom loses an electron, it becomes a
An atom loses electrons to form a cation that is a positively charged ion and one that is attracted towards the negatively charged terminal the cathode. When an atom loses its electrons it becomes negatively charged and is called a negative ion27Jun2022 What does it mean to lose an electron.
 |
| How To Do It Step One The Octet 8 Rule Atoms Will Gain Or Lose Electrons To Have A Total Of 8 Electrons In Their Outer Shell Ppt Download |
When an atom loses an electron it becomes a cation positive ion.
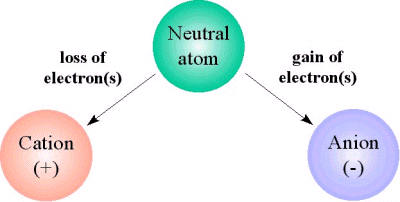
. When an atom loses an electron it becomes an ion. When an atom loses one of its electrons it becomes a positively charged ion. What happens to an atom that loses electron. When an atom looses electrons it becomes a positive ion.
When an atom gainsloses an electron the atom becomes charged and is called an ion. AnswerWhen an atom loses electrons it will lose some of its negative charge and so becomes positively charged. Electron cloud of electrons needed by an oxygen atom to become stable. This can have serious consequences for the atom as it can no longer hold onto its electrons and atoms.
When it gains electrons it becomes negatively charged and is called. What is the charge of an atom if it loses one electron. Whenever electrons are transferred between objects neutral matter becomes charged. When An Atom Loses A Valence Electron It Becomes admin July 31 2022 An atom that loses one or more valence electrons to become a positively charged ion is known.
What happens to an atom that loses electron. When an atom gains an electron it becomes an anion. 2 of electrons needed by a hydrogen atom to become stable. In a nutshell when an atom loses an electron it becomes a positively charged ion.
A hydrogen ion is formed when a hydrogen atom loses an electron and therefore becomes positively charged. Ions formed by the loss of an electron have a positive charge and those formed by gaining an electron have. Chemical formula for water. T or F A substance that releases hydrogen ions in water is a.
12012018 Answer. AnswerWhen an atom loses electrons it will lose some of its negative charge and so becomes positively charged. When an atom loses electrons it will lose some of its negative charge and so becomes positively charged. A positive ion is formed.
Hence option a is correct. What happens to an. T or F An atom that has gained or lost electrons is called an ion. The atom that gains the electron becomes a negatively charged ion.
What happens when a hydrogen atom loses an electron. T or F Water is an example of a compound. This occurs even with individual atoms. The atom that has lost an electron becomes a positively charged ion called a cation while the atom that picks up the.
Atoms are neutral in electric charge because. Both charge and mass. Atoms that lose electrons. A positive ion is formed where.
A positive ion is formed where an atom has more protons than electrons. When an atom gains or loses an electron it becomes an ion. An atom becomes an Ion a if it gains one or more electrons or b if it loses one or more electrons. The atom that loses the electrons becomes a positively charged ion cation while the one that gains them becomes a negatively charged ion anion.
Gaining an electron results in a negative charge so the atom is an anion. The atoms are the smallest unit of matter which consist of centrally located hard mass. How do ions form. However if something happens to make an atom lose or gain an electron then the atom will no longer be neutral.
 |
| Basic Chemistry Atoms And Ions |
 |
| Ionic Bonds What Is An Ion Ppt Download |
 |
| Lesson 5 Basic Chemistry |
 |
| Solved Nel Gallr Uul Luse Electrons Are Called Ions If A Chegg Com |
 |
| What Is An Atom With More Electrons Than Protons Quora |
Post a Comment for "when an atom loses an electron, it becomes a"